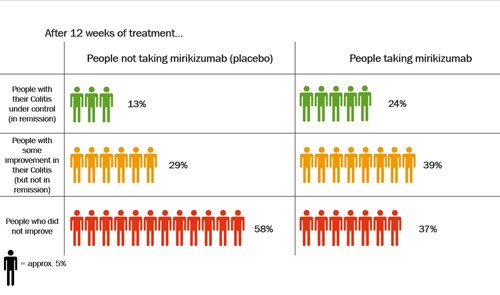
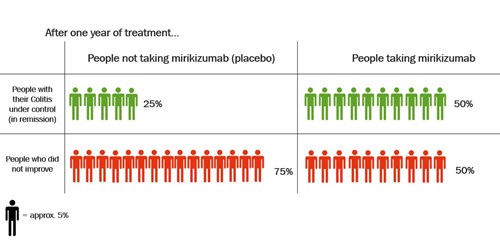
Dose of mirikizumab
You cannot take mirikizumab by mouth because it would be broken down by the gut, which stops it working. Instead, you are given it through a drip to start, and then as an injection under your skin.
You have your first three doses of mirikizumab through a drip into a vein. This is called an intravenous, or IV, infusion. You go to hospital to have this. You should have one infusion every four weeks for a total of three infusions.
If you have had a good response to the first three infusions, you will switch over to having mirikizumab as an injection under your skin every four weeks. This is called a subcutaneous, or SC, injection. You might have this in hospital, or you may be able to give yourself the injection at home. See the section having the injection for more information.
If you have Colitis and you do not have a good response to the first three infusions, your IBD team may suggest that you continue having mirikizumab by infusion for another three doses. If you have a good response after these six infusions, you will switch over to having mirikizumab as an injection. If there is no improvement, your IBD team may suggest stopping mirikizumab and trying a different treatment.
For Crohn’s, if mirikizumab stops working, your IBD team will help to decide the best way to treat you.
Having the infusion
You will have to go to hospital to have the infusion. You can often have it in a day unit, so you will only be there for a few hours. A doctor or nurse will give you the infusion through a drip into one of the veins in your arm.
- For Colitis, you will be given 300mg of mirikizumab over 30 minutes during each infusion.
- For Crohn’s, you will be given 900mg of mirikizumab over 90 minutes during each infusion.
After this, the doctor or nurse will flush your drip through with a solution of saline. Saline is a mix of salt and water. This is to make sure that all the mirikizumab goes into your vein, and none is left in the drip. The flush should not be painful, but it may feel a bit cold.
After each of your infusions your doctor or nurse will probably keep an eye on you for up to one hour. This is to make sure that you are not having a reaction to the infusion. Mirikizumab is not likely to affect your ability to drive after your infusion.
Having the injection
Most people will be able to inject themselves with mirikizumab at home. But some people may continue to have their mirikizumab injection in hospital. If you are going to have it at home, you will need to learn how to inject yourself. Your IBD doctor, nurse or pharmacist, or someone from the home delivery team will teach you how to do this. If you prefer, it may be possible for them to teach someone else to give you the injections. This might be a friend or family member.
Mirikizumab injections come ready to use in a pre-filled injection pen.
- For Colitis, the maintenance dose is 200mg every four weeks. To get this dose, you will need to have two injections, one right after the other. You need two injections because the amount of solution needed to make up the full dose is too much to inject under your skin in one go.
- For Crohn’s, the maintenance dose is 300mg every four weeks. To get this dose, you will need to have two injections, one right after the other. You need two injections because the amount of solution needed to make up the full dose is too much to inject under your skin in one go. One injection will contain 100mg, and the other 200mg. These can be injected in either order.
For both Crohn’s or Colitis, do not inject in the exact same spot every time. For example, if your first injection was in your tummy, your second injection could be in a different place in your tummy.
It is important to have both doses together, one immediately after the other. This is so that you have the best chance of mirikizumab working. If, for any reason, you cannot have the second dose straight away, contact your IBD team for advice.
Delivery
Your hospital may arrange for a special delivery company to send your mirikizumab injections to your home. Mirikizumab can only be prescribed by a specialist in the hospital. It is not a medicine that your GP can prescribe for you to pick up from your local pharmacy.
Storage
Keep mirikizumab injections in the fridge, between 2°C and 8°C. Keep them in the original carton to protect them from light. Do not microwave the pens, run hot water over them, or leave them in direct sunlight. Do not shake your pre-filled pen.
If needed, for example if you are travelling, you can keep mirikizumab injections out of the fridge for up to 14 days, or two weeks. You must keep them in the original carton at room temperature, up to 30°C. They must be out of direct sunlight. Do not use the injections if they are left out of the fridge for more than 14 days. Ask your pharmacist to get rid of any unused medicines. Find out more about travelling with medicines in our information on Travelling with Crohn's or Colitis.
Tips on injecting
Pain at the injection site is a common side effect. You may also get redness, itching and swelling. You should expect to feel some pain, but these tips can help to make it easier to manage.
- Let your medicine warm to room temperature
It might be uncomfortable if you inject yourself with mirikizumab straight from the fridge. Take it out of the fridge about 30 minutes before you inject it, so it can warm to room temperature. Do not warm the injection in any other way, such as in hot water or a microwave.
- Apply an ice pack before you inject
You might find it helpful to apply an ice pack to the injection area for two to three minutes before you inject. If you do this, put a thin towel under it or wrap it in a cloth so it does not damage your skin.
- Choose your injection site
The upper thigh or tummy, away from the belly button, are good places for the injection. Avoid any areas where the skin is red, scarred, bruised or hard. Do not use the same place every time.
- Wash your hands and clean the skin at the injection site
Wash your hands with soap and water. Make sure the skin at the injection site is clean before you inject. You can use an alcohol wipe to do this. This is to reduce the risk of infection.
- Use a good injection technique
Place and hold the clear base of the pen flat and firmly against your skin, then unlock it. Listen for the first click; it means the injection has started. Keep pressing and holding the clear base firmly against the skin. When you hear the second click, it means that the injection is complete. Inject the second pen immediately after the first pen. Make sure the second injection is in a different place.
- Use an ice pack after you inject
Some people recommend applying an ice pack or cold damp towel to the area for 10 to 15 minutes after you have injected to help with pain at the injection site. Remember to put a thin towel under the ice pack or wrap it in a cloth.
- Wear loose clothing.
Wear loose clothing to avoid rubbing or pressure on the injection site.
If you are having problems injecting your mirikizumab, ask your IBD team for help.