Fertility
Golimumab has not been found to affect fertility. Speak to your IBD team if you are thinking of trying for a baby.
Pregnancy
Manufacturers advise that people who could get pregnant should use effective contraception while taking golimumab. This is to prevent pregnancy. They recommend carrying on using contraception for at least 6 months after your last injection of golimumab.
There is a moderate amount of research that looks at the use of golimumab during pregnancy. From the information available, golimumab does not seem to cause problems during pregnancy or to babies exposed during pregnancy.
Experts agree that taking golimumab while you are pregnant is probably a low risk. But, there hasn’t been enough research to rule out the possibility completely. To be cautious, drug companies say that golimumab should only be used during pregnancy if needed to keep your condition under control.
During pregnancy, it’s important to keep your condition under control. Stopping golimumab may increase your risk of a flare-up. Your IBD team may advise that the safest option is for you to keep taking it even during pregnancy. Having active Crohn’s or Colitis while pregnant can lead to premature (early) birth, low birth weight and higher rates of miscarriage.
If you take golimumab during pregnancy, your baby may temporarily have a higher risk of getting an infection. It’s important that you tell your baby’s doctors and other health care professionals if you took golimumab during pregnancy. It’s particularly important to tell them before your baby receives any vaccines (see your baby and live vaccines).
Your baby may be more prone to infections if you take azathioprine or mercaptopurine along with anti-TNFs, like golimumab, during pregnancy.
You and your IBD team should discuss whether the benefits of taking Golimumab outweigh any risks to you and your baby. You can find out more in our information on pregnancy and reproductive health.
Your baby and live vaccines
Taking golimumab during pregnancy may affect when your baby can have live vaccines. This includes the BCG for tuberculosis and the rotavirus vaccine. It should not affect the rest of your baby’s vaccination schedule.
If you take golimumab during pregnancy, you may be told that your baby should not have live vaccines until they are a bit older. National guidelines state that you may need to wait until your baby is between 6 and 12 months old. Drug manufacturers that produce golimumab recommend you wait until six months after your last golimumab dose during pregnancy. But sometimes the benefit of giving a live vaccine earlier might be greater than the potential risk.
You must tell your baby’s healthcare team you were taking golimumab while pregnant. Decisions on what vaccines your baby should have and when will be made on an individual basis. Your IBD team and midwife or baby’s healthcare team should be able to help you come to a decision.
When your baby is old enough to have live vaccines, you should take extra care if they have the rotavirus vaccine. Live virus can be shed in the baby’s poo for up to 14 days. Make sure you wash your hands and/or wear gloves when changing their nappy.




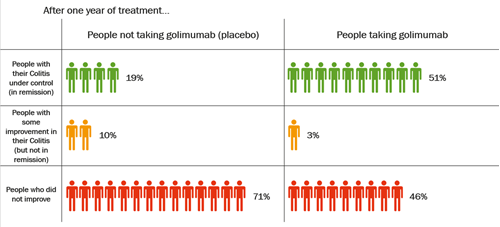
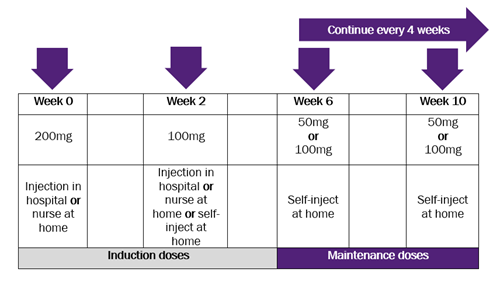
 Become a member: Find out about the benefits of joining our amazing community
Become a member: Find out about the benefits of joining our amazing community