Vedolizumab can help to get your Crohn’s or Colitis under control and keep it under control.
Find out more about how we talk about the effectiveness of medicines.
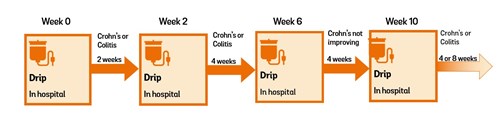
Induction treatment and maintenance treatment
At first, the aim of vedolizumab treatment is to reduce the inflammation in your gut and get your Crohn’s or Colitis under control. This is induction treatment. Once your Crohn’s or Colitis is under control, treatment aims to keep it under control (in remission). This is maintenance treatment.
If you have Crohn’s Disease
One large study looked at vedolizumab as both induction and maintenance treatment for Crohn’s. The study compared vedolizumab given by infusion with placebo (dummy treatment). Some of these people had already been treated with other anti-TNF medicines and some had not.
Getting Crohn’s under control with vedolizumab
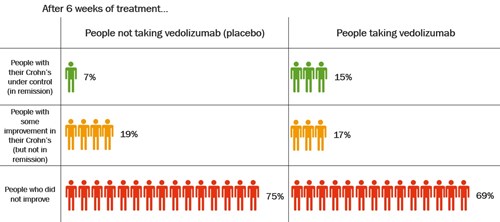
The table shows how well vedolizumab got Crohn’s under control after 6 weeks.
Click to view at full size
After 6 weeks, about twice as many people who took vedolizumab had their Crohn’s under control compared with those who took placebo.
Keeping Crohn’s under control with vedolizumab
People whose disease was under control after induction treatment with vedolizumab continued in the study for up to 1 year. They were given vedolizumab every 8 weeks or placebo.
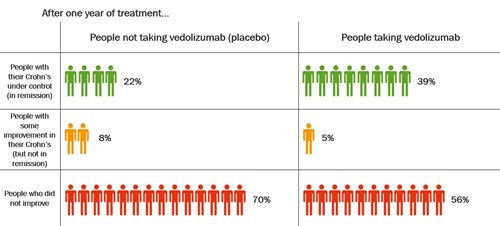
The table shows how well vedolizumab kept Crohn’s under control after 1 year.
Click to view at full size
After 1 year, nearly twice as many people had their Crohn’s under control after taking vedolizumab compared with people who had been taking placebo. About 39% (39 in every 100 people) who took vedolizumab had their Crohn’s under control compared with 22% (22 in every 100 people) who had been taking placebo.
This study looked at vedolizumab given by intravenous infusion. More recent studies using vedolizumab given by subcutaneous injection showed similar effects.
If you have Ulcerative Colitis
These results come from a review that combines several studies. The review compared vedolizumab given by infusion with placebo (dummy treatment). Some of these people had already been treated with other anti-TNF medicines and some had not.
Getting Colitis under control with vedolizumab
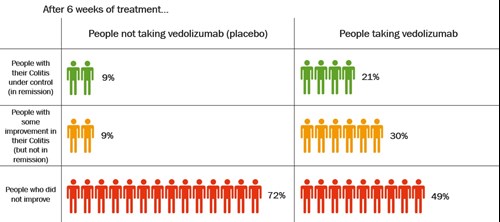
The table shows how well vedolizumab got Colitis under control after 6 weeks.
Click to view at full size
After 6 weeks, more than twice as many people who took vedolizumab had their Colitis under control compared with those who took placebo.
Keeping Colitis under control with vedolizumab
People who had a response to vedolizumab after 6 weeks continued in the study for up to 1 year. They were given vedolizumab every 8 weeks or placebo.
The table shows how well vedolizumab, given every 8 weeks, kept Colitis under control after 1 year.
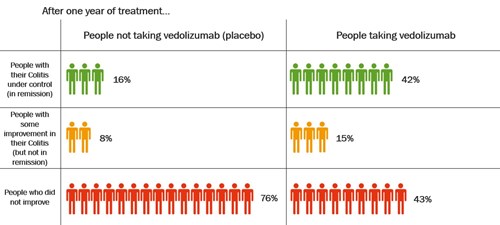
Click to view at full size
After 1 year, more than twice as many people who took vedolizumab had their Colitis under control compared with those who took placebo. About 42% (42 in every 100 people) who took vedolizumab had their Colitis under control compared with 16% (16 in every 100 people) who took placebo.
This review looked at vedolizumab given by intravenous infusion. More recent studies using vedolizumab given by subcutaneous injection showed similar effects.