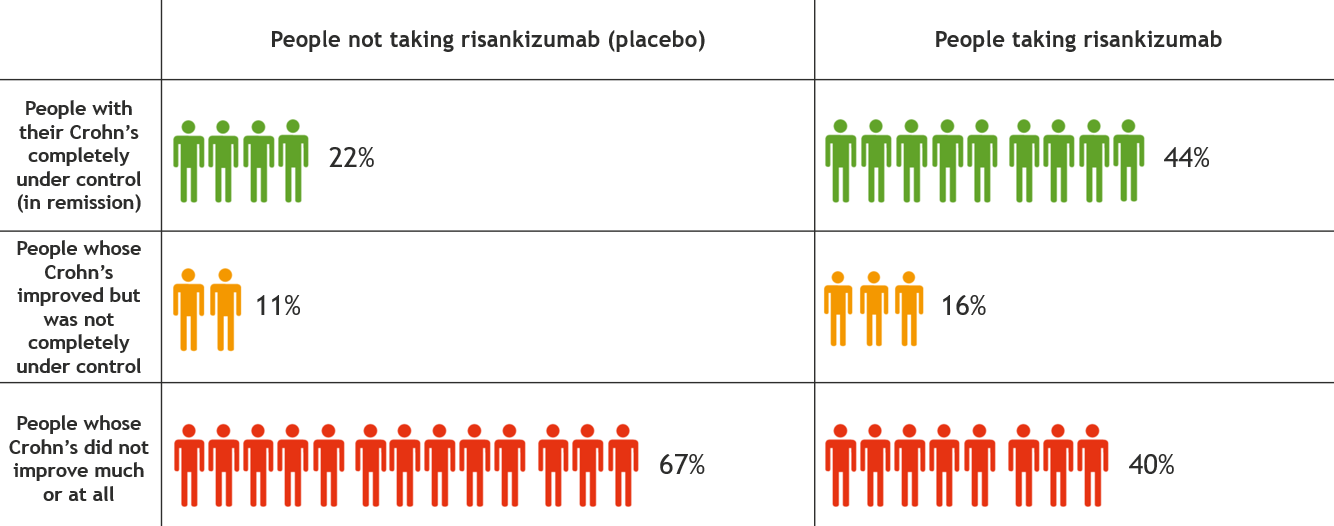
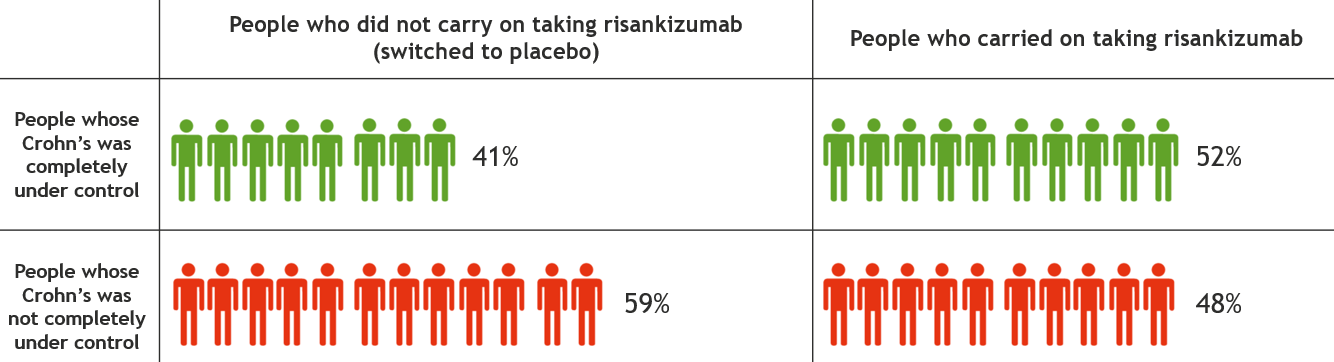
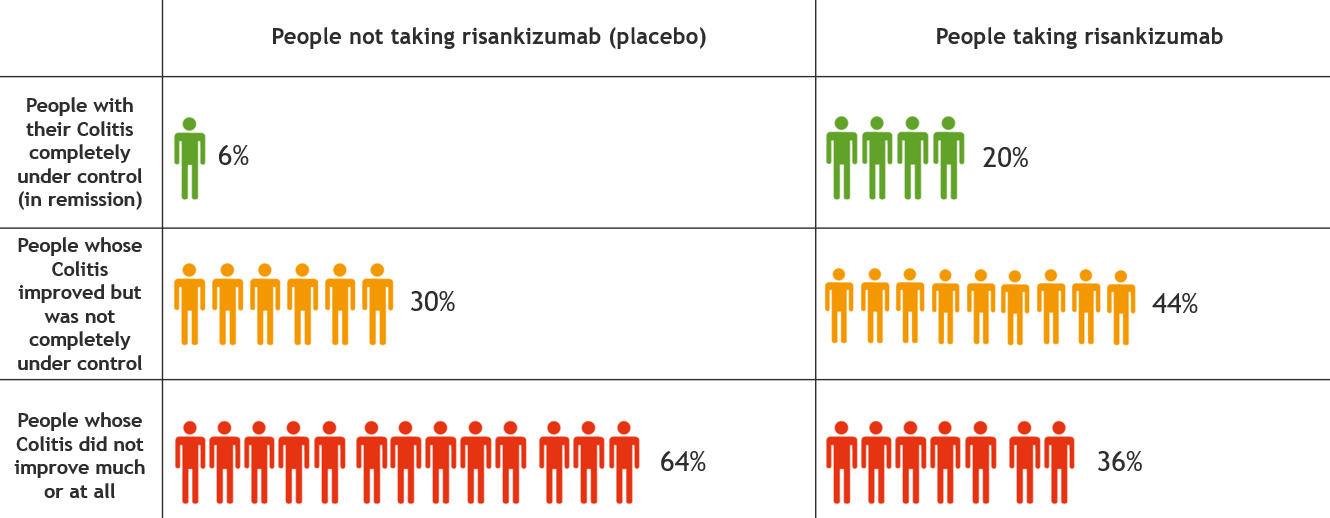
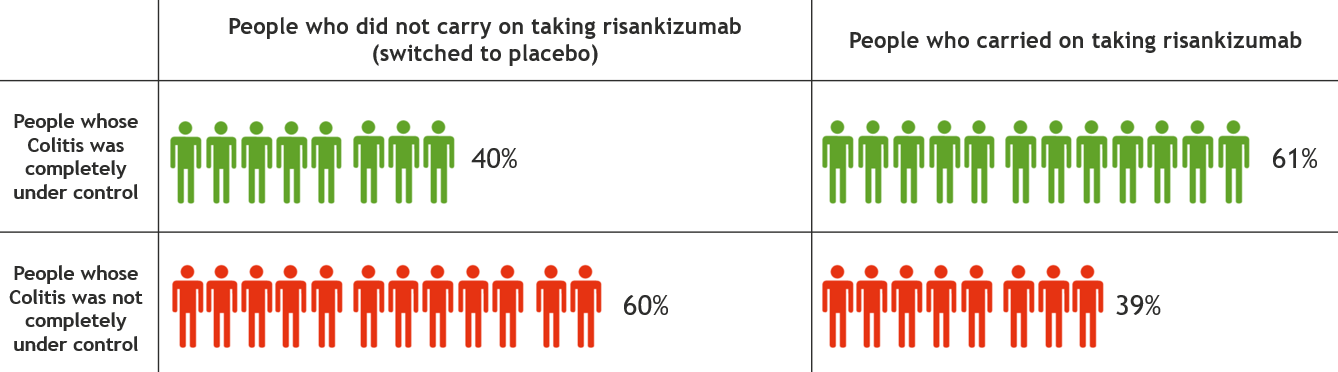
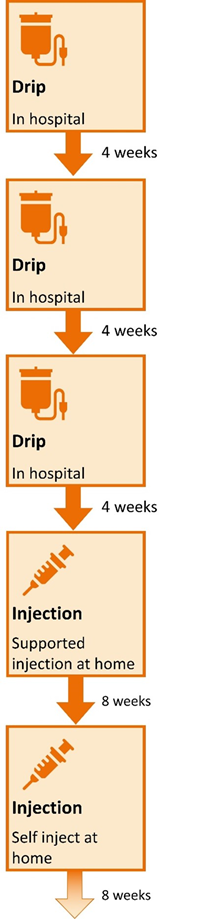
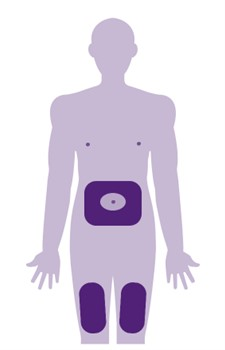
This information is for people with Crohn’s or Colitis who are on risankizumab (Skyrizi) treatment or who are thinking about starting it. Our information can help you decide if this treatment is right for you. It looks at:
- How the medicine works
- What you can expect from the treatment
- Possible side effects
- Stopping or changing treatment
This information might use words you have not heard before. Our page on medical words can help provide an explanation.
This information is about risankizumab in general. It should not replace advice from your IBD team.