Help us improve our information
We need your help to improve our information to better support people with Crohn’s and Colitis. Fill in our short survey to let us know what we're doing well and how we can better meet your needs.
Last full review: September 2022
Currently under review
This information is for people with Ulcerative Colitis who are taking filgotinib (Jyseleca). It is also for anyone who is thinking about starting treatment with filgotinib. This information can help you decide if filgotinib is right for you. It looks at:
This information might use words you have not heard before. Our page on medical words can help provide an explanation.
We need your help to improve our information to better support people with Crohn’s and Colitis. Fill in our short survey to let us know what we're doing well and how we can better meet your needs.
Filgotinib is also known by the brand name Jyseleca.
Filgotinib is a type of medicine called a Janus kinase (JAK) inhibitor. JAKs are proteins that play a part in activating the body’s immune response. This can cause gut inflammation in Ulcerative Colitis. Filgotinib blocks the effects of JAKs, reducing inflammation in the gut.
Tofacitnib is another JAK inhibitor that is used to treat Colitis. You may also hear JAK inhibitors called small molecule drugs.
Filgotinib is used to treat moderate to severely active Ulcerative Colitis in adults. The aim of treatment is to get your Colitis under control and keep it under control.
Your IBD team might suggest filgotinib if standard treatments, a biologic medicine or tofacitinib:
Standard treatments for Colitis include 5-ASAs, steroids, or immunosuppressants such as azathioprine, mercaptopurine or methotrexate. Biologic medicines include adalimumab, golimumab, infliximab, ustekinumab and vedolizumab. You do not need to have tried all of these before considering filgotinib.
Filgotinib is not recommended for use in children. This is because there is not yet any research in people under 18 years.
Filgotinib is not currently recommended to treat adults aged 75 years and older. Older people often have other health conditions, including serious infections. This can make the side effects of filgotinib more serious. There is no research about filgotinib as a treatment for Colitis in this age group.
There are lots of things to think about when you start a new treatment. Your IBD team will discuss your options with you. When thinking about a new treatment you might want to consider the potential benefits, possible risks and the goals of your treatment. Some things to think about include:
Our Appointment guide has a list of questions you might want to ask. It can help you focus on what matters most to you. You might find our information about other medicines and surgery for Colitis helpful.
Use this tool to understand more about potential treatment options that suit your needs. The tool is designed to help you:
Find out more about how we talk about the effectiveness of medicines.
Filgotinib can help to get Colitis under control and keep it under control (in remission). It can also reduce the need for long-term steroid treatment. But it does not work for everyone.
In one clinical trial, people took filgotinib or a placebo for about 1 year. A placebo is a dummy treatment that looks the same but does not have any medicine in it. At the end of the trial, more than three times as many people who took filgotinib were in remission compared with people who had taken a placebo.
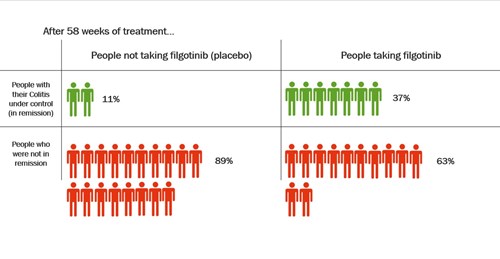
Steroids are usually used to quickly reduce inflammation and control symptoms in people with Colitis. But they should not be taken in the long-term because of their side effects. One of the aims of treatment for Colitis is to keep people off steroids as much as possible.
At the start of the trial between 2 and 4 in every 10 people in the study were taking steroids. The research looked at how many of these people were able to stop taking steroids during the trial. By the end of the trial, more people who took filgotinib were able to stop taking steroids for at least 6 months compared with people who took a placebo.
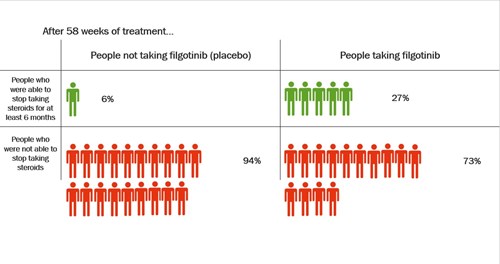
Longer trials are being done to see whether filgotinib continues to work after a year.
Filgotinib has not been compared directly with other treatments for Colitis.
Everyone responds differently to a new medicine. If filgotinib is going to work for you, you will usually start to feel better within about 10 weeks. Some people may take a bit longer to start to feel better. In some people it can take up to 22 weeks (5 to 6 months) to get relief from symptoms. For some people, filgotinib does not relieve their symptoms at all.
Filgotinib can only be prescribed by a specialist IBD team and not your GP.
Filgotinib is taken as a tablet. Each tablet contains 100mg or 200mg filgotinib.
To get symptoms under control (induction treatment):
To keep symptoms under control (maintenance treatment):
Try to take the tablet at a similar time each day. Swallow the tablet whole with a drink of water. Do not split, crush or chew the tablet before swallowing as it may change how much medicine gets into your body. You can take filgotinib with or without food.
Filgotinib tablets contain lactose. You should let your IBD team know if you have been told you have an intolerance to some sugars.
Your IBD team will review your treatment every 6-12 months to check whether it is still the best option for you.
There are a few reasons why you or your IBD team might think about stopping or changing your treatment:
When you start taking filgotinib you might continue to take steroids, aminosalicylates (5-ASAs) and other immunosuppressant medicines such as azathioprine, mercaptopurine or methotrexate. If you are taking steroids when you start filgotinib, you may be able to reduce the dose of steroids if you respond well to filgotinib. Or you may be able to stop taking them altogether. Your IBD team will advise you about this.
It is important that you do not stop taking steroids without speaking to your IBD team.
You should not take biologic medicines or tofacitinib if you are taking filgotinib. If you are taking other immunosuppressant medicines as well as filgotinib, your immune system may be further suppressed.
Taking filgotinib can mean that your body is not able to fight off infections as well as it used to. It can also cause previous viral infections, such as shingles, to return. Before you start filgotinib, your IBD team will ask you some questions and do some tests. This is to make sure your risk from an infection is as low as possible. Tell your IBD team if:
Your IBD team may also ask about any vaccinations you have had. This is to make sure that your vaccinations are up to date before you start filgotinib. Let them know if you are going to have any vaccinations, or you have had a vaccination recently.
Filgotinib may increase blood cholesterol levels in some people. Your IBD team will do a blood test to measure your cholesterol levels before you start treatment. They will measure the levels again after you have been taking filgotinib for about 12 weeks. You may need further checks or treatment to lower your cholesterol levels.
Filgotinib can reduce the levels of white blood cells (lymphocytes, neutrophils) and red blood cells (haemoglobin) in your blood. You should have blood tests before you start treatment to check these. You should also have these levels checked at regular intervals during treatment. Depending on the results your IBD team may recommend that you delay or stop treatment with filgotinib. You will be able to start filgotinib again when the blood levels return to normal.
When you start filgotinib, your IBD team should advise you how and when they will review how well it is working. Your IBD team will ask about your symptoms and any side effects that you may be getting. Your IBD team will also check for any signs of infection.
You will have regular blood tests to check your white blood cells and red blood cells.
It’s important that you attend your appointments and have blood checks to make sure this medicine is prescribed safely.
There are some things that might mean filgotinib is not right for you, or that it could have a higher risk of causing serious side effects.
The UK Medicines Agency (the MHRA) has recommended that all JAK inhibitors, including filgotinib, should only be used in the following people if no other suitable options are available:
The MHRA also recommends that these medicines should be used with caution in people who are at risk of blood clots in their lungs or legs.
If filgotinib is used in people with any of these risk factors, the dose should be reduced.
An increase in the risk of heart attacks and stroke has been seen with tofacitinib (another JAK inhibitor) compared with anti-TNF medicines (such as infliximab and adalimumab). Heart attacks and stroke have also been seen in some people taking filgotinib.
Because of this, filgotinib should only be used in people who are at higher risk of heart disease and stroke if no suitable option is available. Find out more about the risk factors for heart disease and stroke (www.nhs.uk).
Seek urgent medical care if you experience any of the following during treatment:
An increase in the risk of some cancers has been seen with tofacitinib (another JAK inhibitor) compared with anti-TNF medicines (such as infliximab and adalimumab). Some cancers have also been reported in some people taking filgotinib. Because of this, filgotinib should only be used in people who are at higher risk of cancer if no suitable option is available.
Certain types of skin cancer (non-melanoma skin cancer) have been seen in people taking filgotinib. If you are at high risk of skin cancer your doctor may recommend that you have regular skin examinations.
To reduce your risk of skin cancer it is a good idea to:
In some medicines that work in a similar way to filgotinib an increased risk of blood clots in the legs (deep vein thrombosis) or lungs (pulmonary embolism) has been seen. The risk of blood clots in people taking filgotinib is not yet known and is likely to be low. The risk is likely to be higher if you have other factors for blood clots. Risk factors include:
Find out more about the risk factors for blood clots.
When you start taking filgotinib you should be given a Patient Alert Card. It is recommended that you carry this card with you. This is so that anyone treating you will know that you are taking filgotinib.
All medicines can have side effects, but not everyone will get them. Some side effects can happen right away, others may happen later. Some side effects are mild and may go away on their own, or after you stop taking filgotinib. Others may be more serious and could need treatment. Some side effects might mean that filgotinib is not right for you.
Speak to your IBD team if you experience any side effects.
Because filgotinib suppresses your immune system your body might not fight off infections as well as other people. You might get more infections than you are used to. Or if you get an infection, it might last longer or be more serious than usual.
Between 1 and 10 in every 100 people who take filgotinib might get a cold, sore throat, sinus infection or urine (wee) infection. Between 1 and 10 in every 1000 people who take filgotinib might get a more serious infection such as pneumonia or shingles.
Talk to your doctor or get medical help straight away if you get any signs of serious infection such as:
If you get an infection, your IBD team might tell you to stop taking filgotinib while the infection is treated. You should be able to start filgotinib again when the infection is under control.
The most common side effects of filgotinib are listed below. These affect between 1 and 10 in every 100 people who take filgotinib.
This is not a full list of side effects. For more information see the Patient Information Leaflet provided with your medicine or visit medicines.org.uk/emc/.
The safety of any new medicine will continue to be monitored after it has become available for use. This is done through longer term clinical studies and through reporting of side effects. We encourage you to report any side effects to the Medicines and Healthcare Products Regulatory Agency (MHRA). You can do this through the Yellow Card scheme online or by downloading the MHRA Yellow Card app (yellowcard.mhra.gov.uk). This helps collect important safety information about medicines.
If you take filgotinib with other medicines that affect your immune system you may be at increased risk of severe infection. Tell your IBD team, doctor or pharmacist if you take other medicines that affect your immune system. You may take these for other medical conditions.
Some medicines can affect how filgotinib works. These include:
Speak to your IBD team, doctor or pharmacist if you are taking or plan to take any other medicines while you are taking filgotinib. This includes medicines that you buy from a pharmacy or a supermarket. It also includes any herbal, complementary or alternative medicines.
Alcohol is not known to interact with filgotinib. To keep the health risks from drinking alcohol low it is best to stay within the recommended limits.
Your IBD team will check that your vaccinations are up to date before you start treatment with filgotinib. This may include the shingles vaccine and BCG.
You should not have live vaccines while taking filgotinib.
In the UK, live vaccines include:
If someone you live with is due to have a live vaccine, ask your IBD team if you need to take any precautions.
The flu, pneumococcal and COVID-19 vaccines are not live vaccines and are safe to have while you are taking filgotinib.
Animal studies have suggested that filgotinib may affect sperm production and sperm quality. But results from a recent study in humans did not show any difference between filgotinib and dummy treatment (placebo).
There is no evidence that filgotinib affects fertility in people who can get pregnant.
You should not take filgotinib if you are pregnant or are planning to become pregnant.
Let your IBD team know immediately if you are taking filgotinib and you become pregnant or you think you might be pregnant. They will be able to discuss the next steps with you.
There is very little information about the use of filgotinib in pregnancy. But studies in animals suggest that it may cause harm to the unborn baby. Use effective contraception whilst you are taking this medicine and for at least 1 week after you stop taking it if you could get pregnant.
Our information on Reproductive Health can help you decide on the right contraceptive for you.
If you are planning to get pregnant, speak with your IBD team as soon as possible. This will allow time to review your treatment options and make sure your Colitis is controlled as well as possible.
We do not know if filgotinib passes into breast milk or what effect it would have on a baby that is breastfeeding. You should not take filgotinib if you are breastfeeding.
We follow strict processes to make sure our information is based on up-to-date evidence and easy to understand.
Please email us at evidence@crohnsandcolitis.org.uk if:
You can also write to us at Crohn’s & Colitis UK, 1 Bishop Square, Hatfield, AL10 9NE, or contact us through our Helpline: 0300 222 5700

We know it can be difficult to live with, or support someone living with these conditions. But you’re not alone. We provide up-to-date, evidence-based information and can support you to live well with Crohn’s or Colitis.
Our helpline team can help by:
Providing information about Crohn’s and Colitis.
Listening and talking through your situation.
Helping you to find support from others in the Crohn’s and Colitis community.
Providing details of other specialist organisations.
Please be aware we’re not medically or legally trained. We cannot provide detailed financial or benefits advice or specialist emotional support.
Please contact us via telephone, email or LiveChat - 9am to 5pm, Monday to Friday (except English bank holidays).
If you need specific medical advice about your condition, your GP or IBD team will be best placed to help.
Would you like to save the changes made to this page?
Your details were successfully saved.
