Help us improve our information
We need your help to improve our information to better support people with Crohn’s and Colitis. Fill in our short survey to let us know what we're doing well and how we can better meet your needs.
Last updated: April 2025
Last full review: September 2024
Next review date: September 2027
Adalimumab is a common treatment for people with Crohn’s and Colitis. This information is for people who take this medicine or are thinking about taking it.
Our information can help you decide if this treatment is right for you. It looks at:
This information might use words you have not heard before. Our page on medical words can help provide an explanation.
This information should not replace advice from your healthcare professional. Talk to your IBD team or read the leaflet that comes with your medicine for more details. You can also find out about your medicine at the Electronic Medicines Compendium.
This information about adalimumab is also available as a Quick Guide. This is a summary of the full information. You might find this useful if you are finding the full information difficult to read and take in. The Quick Guide can easily be printed off to read later.
We need your help to improve our information to better support people with Crohn’s and Colitis. Fill in our short survey to let us know what we're doing well and how we can better meet your needs.
Adalimumab is known by several brand names, including:
Your medicine will be prescribed by brand name.
Humira was the first brand of adalimumab. Amgevita, Hyrimoz, Idacio, Imraldi and Yuflyma work in the same way but are known as ‘biosimilars’. This means they are similar to Humira and have the same treatment effects. There may be some slight differences between brands, such as:
The brand you are given will not affect your treatment, but you may want to ask your healthcare professional which brand you take. For more about biosimilars see our information on biologic medicines.
Adalimumab is my little bit of magic in a pen: I have been on this treatment over 3 years.
Thea
Living with Crohn's
Adalimumab belongs to a group of medicines called biologic medicines. These medicines are made by a biological rather than a chemical process. They are made in a lab from living cells.
Adalimumab is a man-made antibody-based medicine. An antibody is a protein that is part of your natural defences. Adalimumab targets a protein in the body called tumour necrosis factor-alpha, known as TNF-alpha. TNF-alpha is naturally produced by your body and helps fight off infections. Too much TNF-alpha can damage the cells that line the gut. This may partly be the cause of gut inflammation for people with Crohn’s or Colitis. Adalimumab binds to TNF-alpha, blocking its harmful effects. This can reduce inflammation and help to relieve symptoms.
Adalimumab is sometimes called an ‘anti-TNF’ medicine. Other anti-TNF medicines are:
Adalimumab is used to treat adults and children from 6 years old with:
It may be given to you if other treatments, such as steroids, azathioprine, mercaptopurine or methotrexate:
Adalimumab may also:
The aim of using this medicine is to try to get your condition under control and keep it under control. This is known as being in remission. Remission is when you feel better because your Crohn's or Colitis is well-controlled. During this time, medical tests, such as blood tests or endoscopy, show your gut is less affected. Your symptoms, such as diarrhoea, abdominal pain or fatigue, will improve. However, some symptoms, like fatigue, may not go away completely. Keeping your Crohn’s or Colitis under control is good for your long-term health. It lowers your risk of complications and the need for surgery.
Adalimumab is also used to treat:
You may be given a choice of taking adalimumab or another biologic medicine. Our information on medicines for Crohn’s and Colitis can help you decide.
There’s lots to think about when you start a new medicine. Your IBD team will talk to you about your options. For new medicines, you might want to think about what you’d like to get out of treatment and what the pros and cons might be. Some things to think about include:
You could use our medicine tool to help you think about your options. Our appointment guide also has a list of questions you might want to ask your IBD team. We also have information on other medicines or surgery for Crohn’s or Colitis.
Use this tool to understand more about potential treatment options that suit your needs. The tool is designed to help you:
Adalimumab can help to get your Crohn’s or Colitis under control and keep it under control.
Find out more about how we talk about the effectiveness of medicines.
The information below shows the results of clinical trials that looked at how effective adalimumab is. To find this out, scientists compared people who took adalimumab with people who took a placebo. A placebo is a substance that looks the same as the treatment but does not have any medicine in it.
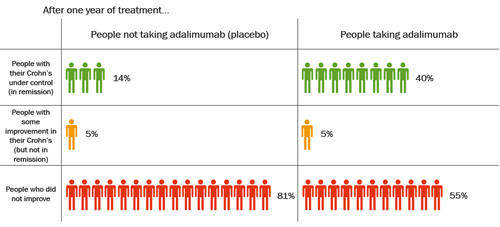
The table below shows data from clinical trials of adalimumab in adults with moderate to severely active Crohn’s Disease.
After one year of treatment with adalimumab, an average of 4 in every 10 people, or 40%, were in remission. Of those who took a placebo, an average of less than 2 in every 10 people, or 14%, were in remission. These statistics show that more than twice as many people had their Crohn’s under control after taking adalimumab for one year, compared with people who had not been taking adalimumab. But adalimumab does not work well for everyone with Crohn’s.
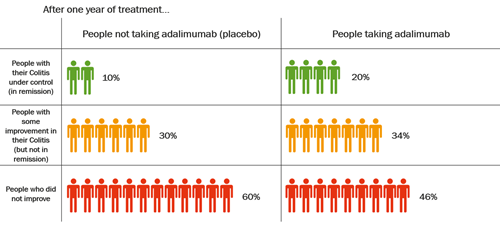
The table below shows data from clinical trials of adalimumab in adults with moderate to severely active Colitis.
After one year of treatment with adalimumab, an average of 2 in every 10 people, or 20%, were in remission. Of those who took a placebo, an average of 1 in every 10 people, or 10%, were in remission. This shows that twice as many people had their Colitis under control after taking adalimumab for one year, compared with people who had not been taking adalimumab. But adalimumab does not work well for everyone with Colitis.
Everyone responds differently when taking a new medicine. You may feel better soon after starting treatment. It may take up to 12 weeks if you have Crohn’s, or up to eight weeks if you have Colitis.
Adalimumab does not work for everyone. Your IBD specialist will check how well it is working for you.
Adalimumab is given as an injection under the skin. Adalimumab cannot be taken as a tablet. This is because if you swallowed adalimumab, it would be broken down by your gut before it could start working.
Your IBD team will monitor your treatment. Your first dose will usually be given to you in hospital, or by a trained nurse at home. You will then be trained to inject it yourself. If you prefer, it may be possible for someone else, such as a family member, to be trained to give you the injections.
Adalimumab for adults comes ready to use in either a pre-filled syringe or a pre-filled injection ‘pen’. For the injection pen, the needle is inside the device and may not be seen. The syringes and pens both come in a pack. Each pack contains an alcohol pad to clean your skin before you inject.
Adalimumab for children comes ready to use in either a pre-filled syringe, a pre-filled injection ‘pen’ or in a small bottle, known as a vial. For the injection pen, the needle is inside the device and may not be seen. The vial is designed to fit onto a syringe using an adapter. Only the brand Idacio comes in a vial. The syringes or pens come in a pack. Each pack contains an alcohol pad to clean your skin before you inject.
Adalimumab will usually be sent to your home by a special delivery company. You cannot pick it up from your local pharmacy.
Keep adalimumab in the fridge.
If you are travelling, you can keep your adalimumab out of the fridge. If you do keep it out of the fridge it must be kept out of the sunlight and stored at room temperature, or below 25°C. Your medicine can be kept this way for up to:
Ask your pharmacist to get rid of your adalimumab if it is not used within this time.
Do not put your medicine back in the fridge once it has been kept at room temperature.
Always check the leaflet that comes with your medicine.
Find out more about travelling with medicines.
Make sure you know how your injection device works.
Different brands use different methods. For some, you will need to pinch your skin before you inject. For others, you may need to press a button to inject the medicine. Check the leaflet that comes with your medicine.
Pain at the injection site is a common side effect. You may get redness, itching or swelling. These tips might help to make the pain easier to manage:
If you still have problems with injecting, ask your IBD team for help or read the leaflet that came with your medicine.
The thought of injecting myself filled me with dread, but it was so easy and is now just a part of my life that I don’t even think about!
Karen
Living with Crohn's
Your first injection will be a higher dose. You may need more than one injection for this. This is because the amount of solution needed to make up the higher dose is too much to inject in one go.
The first few doses are known as induction doses. These can help you respond to the medicine quickly. After your first dose, you will have another induction dose two weeks later. If you respond well, you will usually have maintenance dosing every two weeks.
Some people may need a quicker response to their medicine. To help with this, their induction dose may be increased. Increasing the dose may increase the risk of side effects.
If adalimumab does not work for you, or it becomes less effective, your doctor may suggest some changes to your dosing schedule. These changes may depend on your symptoms and blood test results. Speak to your IBD team if you think your medicine is not working as well as it should. Do not make any dose changes unless your IBD team tells you to.
Talk to your IBD team or look at the leaflet that comes with your medicine to see how much you should take.
If adalimumab works well, you will continue your treatment for a few months. After this, your IBD team may check if this medicine is still helping you. If you are responding well and it is not causing you any serious side effects, then you will continue treatment. Your IBD team should assess you at least every eight to twelve months to check adalimumab is still right for you. Adalimumab is safe to take for a long time and many people use it for many years.
Your IBD team may think it is right to stop or change your treatment if:
You have a right to take part in decisions about your treatment. Tell your IBD team what matters most to you. This will help them give you the information and support you need. Our guide to appointments can help you have these conversations. Do not stop taking your medicine unless your IBD team says it is ok. If you must stop taking this medicine but are still unwell, you may be able to try a different biologic. See our information on biologic medicines.
You may take adalimumab with other medicines for your Crohn’s or Colitis. These include:
Taking more than one medicine is known as combination therapy. For more on this, see our information on taking medicines.
Taking a combination of adalimumab with methotrexate or a thiopurine, such as azathioprine, may be more effective. This can reduce the risk of adalimumab becoming less effective over time. Speak to your IBD team about the risk of side effects with combination therapy. You should decide together what the best treatment option is for you.
Your IBD team will check if adalimumab is right for you. This will involve asking about any pre-existing conditions and having some tests. This may include blood tests and imaging, such as an X-ray.
Having treatment that affects your immune system can mean that your body may not be able to fight off infections as well as it used to. Before you start adalimumab, your IBD team may ask you some questions and do some tests. This is to make sure your risk of an infection is as low as possible. Tell your IBD team if:
Tell your IBD team if:
You will be regularly monitored for any side effects and have checks to make sure that adalimumab is right for you. This may sometimes include blood tests. Regular checks can catch problems at an early stage. Tell your IBD team if you notice any new symptoms or side effects.
Blood tests may be used to check the levels of adalimumab and antibodies in your blood. This helps your IBD team to see if adalimumab is working or if your dose needs changing.
You need to attend your appointments and have blood tests so that your adalimumab is given to you safely.
Some people may feel dizzy or have blurred vision after taking adalimumab. Do not drive, ride a bike or use any tools or machines if you feel dizzy or tired after taking this medicine.
This medicine affects the way your immune system works. Your immune system can fight off infections, but it may not be as strong as before. You may find that you get more infections or that they affect you more than they used to. Tell your IBD team if you have signs of an infection. This might include a sore throat, fever, or any new symptoms that concern you. Your IBD team may advise you to wait until you feel better before having adalimumab. Less often, serious infections can be a side effect of adalimumab. See the section below on side effects.
Although your risk of infections may be higher when taking adalimumab, it should not stop you from living life as before. See our information on immunosuppressant precautions to find out some practical things you can do to reduce your risk.
Take care in the sun
You may be more at risk of skin cancer. You should practice good sun safety, this includes:
The NHS website has more tips for staying safe in the sun.
Cervical cancer screening was previously known as the smear test. Changes to the cells in the cervix can be caused by the human papillomavirus, known as HPV. If you are immunosuppressed, you are more likely to catch HPV, which can cause these changes. Taking adalimumab does not automatically mean you need to be tested more regularly than other people. However, if you are HPV positive, you may need to go for screening more often. There is no evidence linking cervical cancer to the use of adalimumab or other biologics. All women and people with a cervix between the ages of 25 and 64 should go for regular cervical screening. You will get a letter from your GP surgery inviting you to make an appointment.
All medicines can have side effects, but not everyone experiences them. Having certain side effects might mean that adalimumab is not right for you.
Speak to your IBD team if you get any side effects.
Some people might get serious side effects that need urgent treatment. These do not happen often, but it is important to know what to look out for.
Some people taking adalimumab might have an allergic reaction. Severe allergic reactions are rare. Life-threatening reactions are even more unusual.
Contact NHS 111 or call 999 straight away if you think you are having an allergic reaction.
Signs to look out for include:
Your heart starts beating very fast
After an allergic reaction has been treated, contact your IBD team to let them know what happened.
If you take adalimumab, you may get infections more easily. This is because adalimumab can affect your immune system. You might get more infections than you are used to. Infections may last longer or be more serious than usual. You may also be more likely to get an opportunistic infection. An opportunistic infection is an infection that happens more often or is more severe in someone with a weakened immune system. Opportunistic infections can include infections like tuberculosis, shingles and thrush.
Tell your doctor or IBD team immediately if you develop symptoms of an infection.
The list below are symptoms to watch out for in yourself and in others:
Symptoms of serious side effects do not happen often, but it is important to know what to look out for. Tell your doctor or IBD team immediately if you develop:
More than 1 in every 10 people taking adalimumab may have:
This medicine may not be right for you if you have previously had cancer. You must tell your doctor if you have a bump or open sore that is not healing.
For a full list of side effects see the Patient Information Leaflet provided with your medicine or visit the Electronic Medicines Compendium website.
You should report any side effects to the Medicines and Healthcare Products Regulatory Agency, also known as the MHRA, through the Yellow Card scheme. Your doctor should also report it. You can report any side effects on the MHRA website.
I have to cope with certain side effects such as pain at the site of injecting and flu-like symptoms such as headache and general achiness but this usually subsides within a couple days. Don’t be scared – it is easier than it seems at first.
Emily
Living with Ulcerative Colitis
Adalimumab is often taken alongside other medicines safely. See the earlier section Taking adalimumab with other Crohn’s or Colitis treatments.
However, adalimumab may interact with some medicines. Speak to your doctor or pharmacist if you are taking, or plan to take any other medicines. This includes:
Do not take medicines that contain anakinra or abatacept. These medicines are commonly used for rheumatoid arthritis.
When starting adalimumab, you should be given a patient reminder card. This contains important safety information about adalimumab. You should show this card to any doctor, dentist or healthcare professional that is treating you. Always carry this card with you while you are taking adalimumab. If you stop taking adalimumab, you should still carry this card for up to 70 days after your last dose.
Live vaccines contain weakened live strains of viruses or bacteria. You should not have live vaccines if you are immunosuppressed. This is because the weakened virus or bacteria could reproduce too much and cause a serious infection.
You should not have live vaccines while taking adalimumab.
Ask your IBD team to make sure your vaccinations are up to date before you start adalimumab. Talk to your IBD team if you are planning to travel and need vaccinations.
In the UK, live vaccines include:
There is a small risk that people who have recently had a live vaccine could pass on the virus or bacteria to close contacts who are immunosuppressed. This could then cause an infection.
For most of the live vaccines used in the UK, the virus or bacteria is not passed on to contacts. You can reduce the risk by following some simple steps, such as:
Talk to your IBD team if someone you live with is due to have a live vaccine and you have any concerns.
It is safe to have non-live vaccines when you are taking adalimumab.
Everyone with Crohn’s or Colitis taking a biologic medicine should be invited to have the flu vaccine every year. You may be advised to have the pneumococcal vaccine. You are also eligible for all doses of COVID-19 vaccination. These are not live vaccines.
People aged 50 years or older who are severely immunosuppressed also qualify for the shingles vaccine. This includes people taking a biologic, such as adalimumab. A non-live shingles vaccine, Shingrix, is available.
Adalimumab is not thought to affect male or female fertility. If you do not want to get pregnant you should use contraception.
Your contraception will not be affected by adalimumab and will work as normal. This includes:
However, if adalimumab makes you vomit, contraceptive pills may not be as effective and may not protect you from pregnancy. If you are sick, check the information that comes with your contraceptive or speak to a pharmacist.
Speak to your IBD team if you are offered or are taking adalimumab and want to start a family. They can help you make an informed decision about your care and your baby's safety.
Do not stop taking your medicine without talking to your doctor first.
Stopping your medicine may increase your risk of a flare-up. Having active Crohn’s or Colitis can increase the risk of pregnancy complications, such as:
This is why it is important to keep your condition under control during pregnancy.
Adalimumab is generally considered safe to take during pregnancy. Research shows that it is not likely to affect your pregnancy or harm an unborn baby. Long-term health, infection rates and development do not appear to be affected in children with a parent who took medicines like adalimumab, during pregnancy. This includes mothers who took these medicines until birth.
It is possible for adalimumab to cross the placenta and enter your baby’s blood. This may mostly happen in mid to late pregnancy, during the late second and third trimester. To be cautious, drug companies advise using contraception to prevent pregnancy while taking adalimumab and for at least five months after your last dose. They also suggest that adalimumab should only be used during pregnancy if needed to keep your condition under control.
However, many people will be advised by their healthcare professionals to continue taking adalimumab throughout their pregnancy. This can help manage their condition effectively and reduce flare-ups. Flare-ups can increase the risk of pregnancy complications.
If your Crohn’s or Colitis is well controlled, your IBD team may advise you to take adalimumab for the first six months of your pregnancy only. This aims to reduce exposure to your baby. If your condition is not well controlled, your IBD team may recommend you take adalimumab throughout your pregnancy.
Contact your IBD team straight away if you are on adalimumab and find out you are pregnant. Do not stop taking your medicine until you have spoken to your healthcare professional.
Tell your baby’s healthcare team if you took adalimumab during your pregnancy. If you did, you may be told that your baby should not have live vaccines until they are a bit older. This includes the rotavirus vaccine and the BCG vaccine for tuberculosis. The BCG vaccine is not routinely given as part of the NHS vaccination schedule but is sometimes recommended. For these vaccinations, you may need to wait until your baby is between five and 12 months old or until adalimumab cannot be found in the baby’s blood. But sometimes the benefit of giving a live vaccine earlier may be greater than the potential risk.
Taking adalimumab during pregnancy should not affect the rest of your baby’s vaccination schedule. You might want to discuss this with your IBD team and your baby’s healthcare team. Decisions on what vaccines your baby should have, and when, will be made on an individual basis. Your IBD team and midwife or baby’s healthcare team should be able to help you make a decision.
If you take adalimumab, you should take extra care if your baby has the rotavirus vaccine. Live virus can be shed in the baby’s poo for a few weeks. Make sure you wash your hands or wear gloves when changing their nappy.
There is some evidence that your baby may be more prone to infections if you take azathioprine or mercaptopurine along with other anti-TNF medicines, like adalimumab during pregnancy.
Discuss the risks and benefits of taking adalimumab while you are pregnant with your IBD team. You can also find out more in our information on pregnancy and reproductive health.
You can take adalimumab while breastfeeding. Speak to your IBD team if this is something you are thinking of doing.
Experts agree that breastfeeding while on adalimumab is unlikely to be harmful to your baby. Studies of babies that were breastfed by people taking adalimumab have shown:
• Normal growth
• Normal development
• Normal rates of infection
Some studies have found small amounts of adalimumab in breast milk, but it has not been found in breastfed babies. Adalimumab cannot be taken by mouth because it is broken down and destroyed in the gut. Adalimumab in breast milk is also likely to be broken down in your baby’s gut, so only very small amounts may be absorbed by your baby. Speak to your IBD team if you are worried.
Find out more information about postnatal care and breastfeeding.
You can drink alcohol while you are on adalimumab. But, just like everyone else, you should follow NHS guidelines on counting your weekly alcohol units to reduce general health risks.
Taking medicines and managing side effects can be difficult. We understand and we’re here to help. Our Helpline can answer general questions about treatment options and can help you find support from others with the conditions.
Your IBD team are also there to help. You can talk to them about your dosage, how they’ll be monitoring you and what other options there might be. You should also get in touch with your IBD team if you have any new symptoms or side effects.
It can take time to find the medicine that’s right for you. Don’t be afraid to ask questions and seek out extra support when you need it.
This information is general and does not replace specific advice from your health professional. Talk to your GP or IBD team for information that’s specific to you.
We’re here for you whenever you need us. Our information covers a wide range of topics. From treatment options to symptoms, relationship concerns to employment issues, our information can help you manage your condition. We’ll help you find answers, access support and take control.
All information is available on our website.
Our helpline team provides up-to-date, evidence-based information. You can find out more on our helpline web page. Our team can support you to live well with Crohn’s or Colitis.
Our Helpline team can help by:
You can call the Helpline on 0300 222 5700. You can also visit our livechat service. Lines are open 9am to 5pm, Monday to Friday, except English bank holidays.
You can email helpline@crohnsandcolitis.org.uk at any time. The Helpline will aim to respond to your email within three working days.
You can find support from others in the Crohn’s and Colitis community through our virtual social events. There may also be a Local Network in your area offering in-person social events. Visit our Crohn’s and Colitis UK in your area webpage to find out what is available.
This closed-group Facebook community is for anyone affected by Crohn’s or Colitis. You can share your experiences and receive support from others. Find out more about the Crohn’s & Colitis UK Forum.
There are many benefits to becoming a member of Crohn’s & Colitis UK. One of these is a free RADAR key to unlock accessible toilets. Another is a Can’t Wait Card. This card shows that you have a medical condition. It will help when you are out and need urgent access to the toilet. See our membership webpage for more information. Or you can call the Membership Team on 01727 734465.
Our Medicine Tool is a simple way to compare different medicines for Crohn’s or Colitis. You can see how medicines are taken, how well they work, and what ongoing checks you need. You can find out more on our Medicine Tool webpage.
The Medicine Tool can help you:
Always talk to your IBD team before stopping or changing medicines.
We follow strict processes to make sure our information is based on up-to-date evidence and is easy to understand. We produce it with patients, medical advisers and other professionals. It is not intended to replace advice from your own healthcare professional.
You can find out more on our website.
We hope that you’ve found this information helpful. Please email us at evidence@crohnsandcolitis.org.uk if:
You can also write to us at Crohn’s & Colitis UK, 1 Bishops Square, Hatfield, Herts, AL10 9NE. Or you can contact us through the Helpline on 0300 222 5700.
We do not endorse any products mentioned in our information.

We know it can be difficult to live with, or support someone living with these conditions. But you’re not alone. We provide up-to-date, evidence-based information and can support you to live well with Crohn’s or Colitis.
Our helpline team can help by:
Providing information about Crohn’s and Colitis.
Listening and talking through your situation.
Helping you to find support from others in the Crohn’s and Colitis community.
Providing details of other specialist organisations.
Please be aware we’re not medically or legally trained. We cannot provide detailed financial or benefits advice or specialist emotional support.
Please contact us via telephone, email or LiveChat - 9am to 5pm, Monday to Friday (except English bank holidays).
If you need specific medical advice about your condition, your GP or IBD team will be best placed to help.
Would you like to save the changes made to this page?
Your details were successfully saved.
